Hydrogen from formic acid
Prof. Emiliano Cortés and his team from the Nanoinstitute at Ludwig-Maximilians-Universität have developed a crystal that produces hydrogen highly efficiently from formic acid and sunlight.

Harvesting the sun's energy is still not easy. "The sunlight arrives on earth 'diluted'. The energy per area is comparatively low," explains Cortés. Solar systems compensate for this over large areas. Cortés is approaching it from the other direction: with his team at LMU's Nano Institute, he is developing plasmonic nanostructures that produce hydrogen from formic acid with the help of solar energy.
Emiliano Cortés has now developed a two-dimensional supercrystal together with Dr. Matías Herrán and cooperation partners from the Free University of Berlin and the University of Hamburg. It consists of just a single layer of nanoparticles and produces hydrogen from formic acid using sunlight. Formic acid is a colorless, corrosive liquid that is soluble in water and is often used by living organisms in nature for defense purposes. The Munich researchers have misappropriated the substance and combined it with their crystal. "The combination is so outstanding that it holds the world record for hydrogen production using sunlight," says Cortés. "Our experiments are feasibility studies and formic acid is a world record material for the use of solar energy."
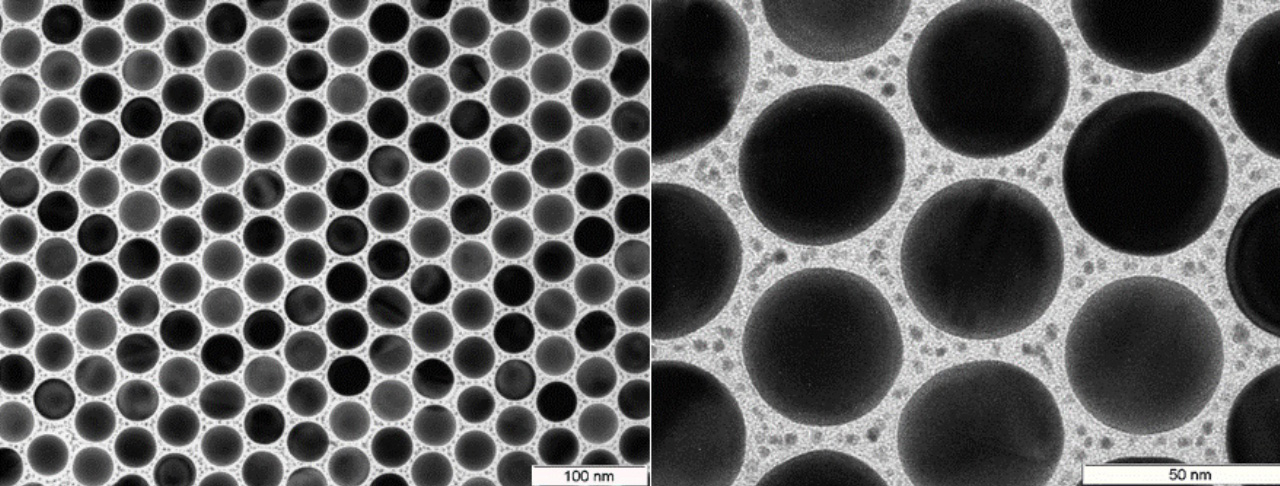
The image from the transmission electron microscope shows large golden nanoparticles, in between the smaller platinum particles for the catalysis of formic acid. (c) Dr. Florian Schulz, University of Hamburg
Nano-hotspots unleash catalysis power
Cortés and Herran use two metals in nano format for their supercrystal. "We first produce particles of 10-200 nanometers from gold, a plasmonic metal," explains Herrán. "At this size, visible light interacts strongly with the gold electrons and causes them to vibrate resonantly." As a result, the nanoparticles capture more sunlight and convert it into extremely high-energy electrons. "This creates strong local electric fields, known as hotspots," says Herrán.

Prof. Emiliano Cortés in the lab.
The hotspots form between the gold particles. This gave Cortés and Herran the idea of placing platinum nanoparticles in the gaps. "In the hotspots, we get them to convert formic acid into hydrogen," explains Herrán. With a hydrogen production rate - starting from formic acid - of 139 millimoles per hour and per gram of catalyst, the photocatalytic material currently holds the world record for hydrogen production with sunlight.
Nowadays, hydrogen is primarily produced from fossil fuels, above all natural gas. "By combining plasmonic and catalytic metals, we are advancing the development of potent photocatalysts for industry, for example the conversion of CO2 into usable substances," explain Cortés and Herrán.
Original publication:
Matías Herrán, Sabrina Juergensen, Moritz Kessens, Dominik Hoeing, Andrea Köppen, Ana Sousa-Castillo, Wolfgang J. Parak, Holger Lange, Stephanie Reich, Florian Schulz, Emiliano Cortés
Plasmonic Bimetallic Two-Dimensional Supercrystals for H2 Generation
Nature Catalysis, 2023.
DOI: 10.1038/s41929-023-01053-9









